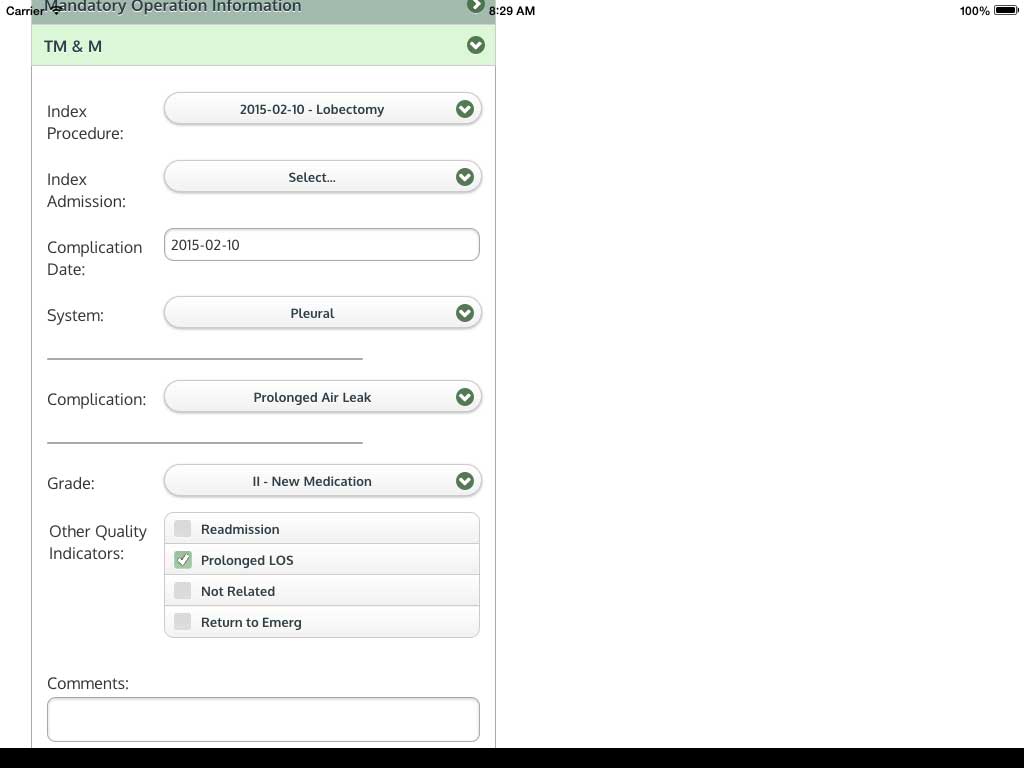
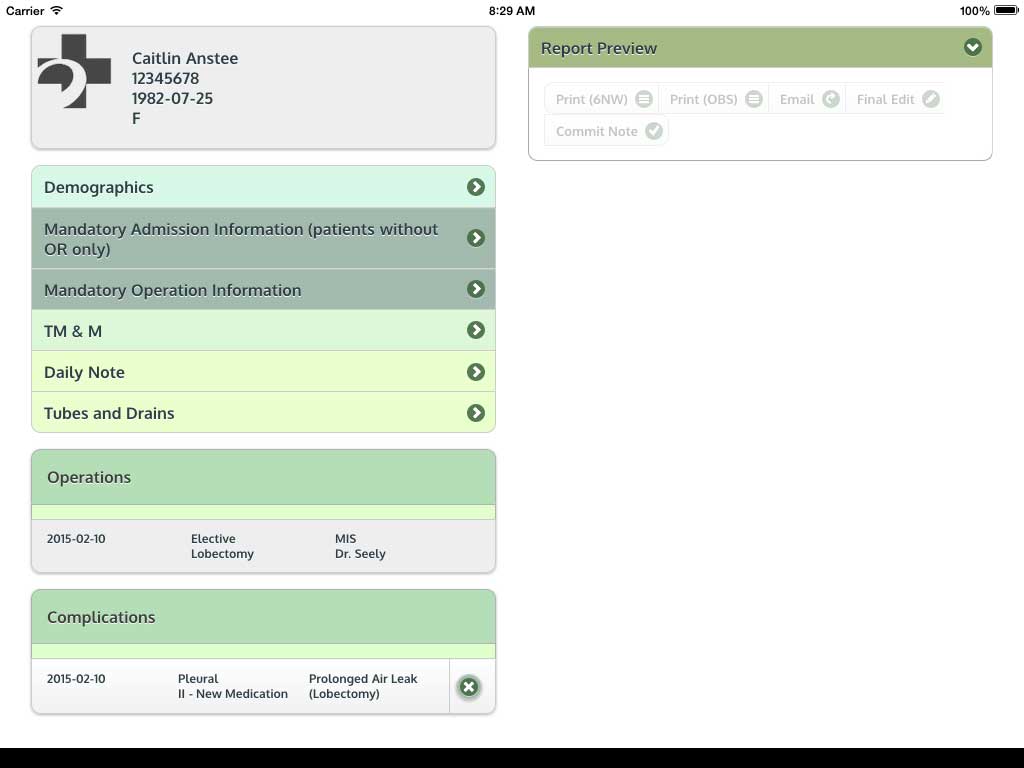
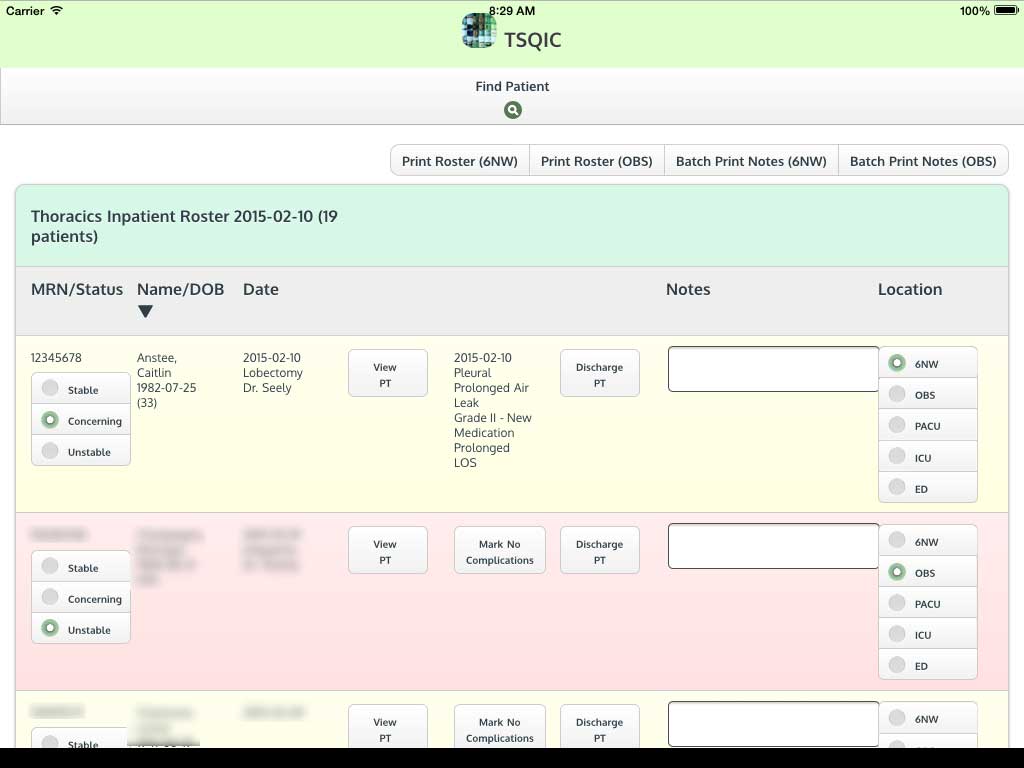
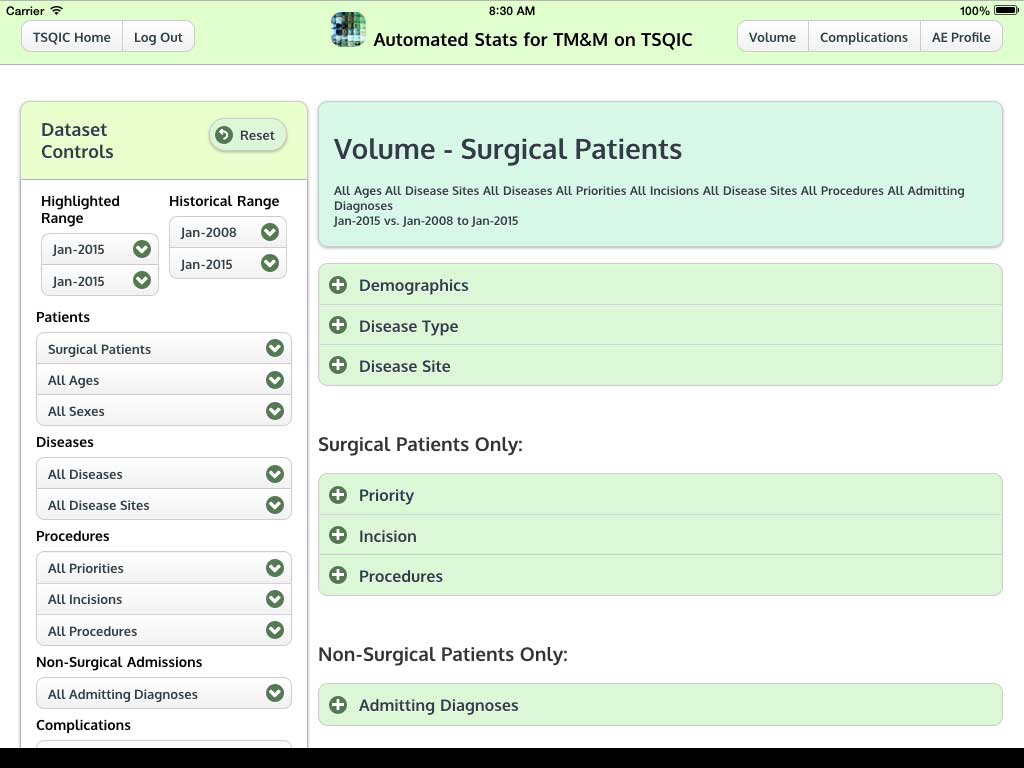
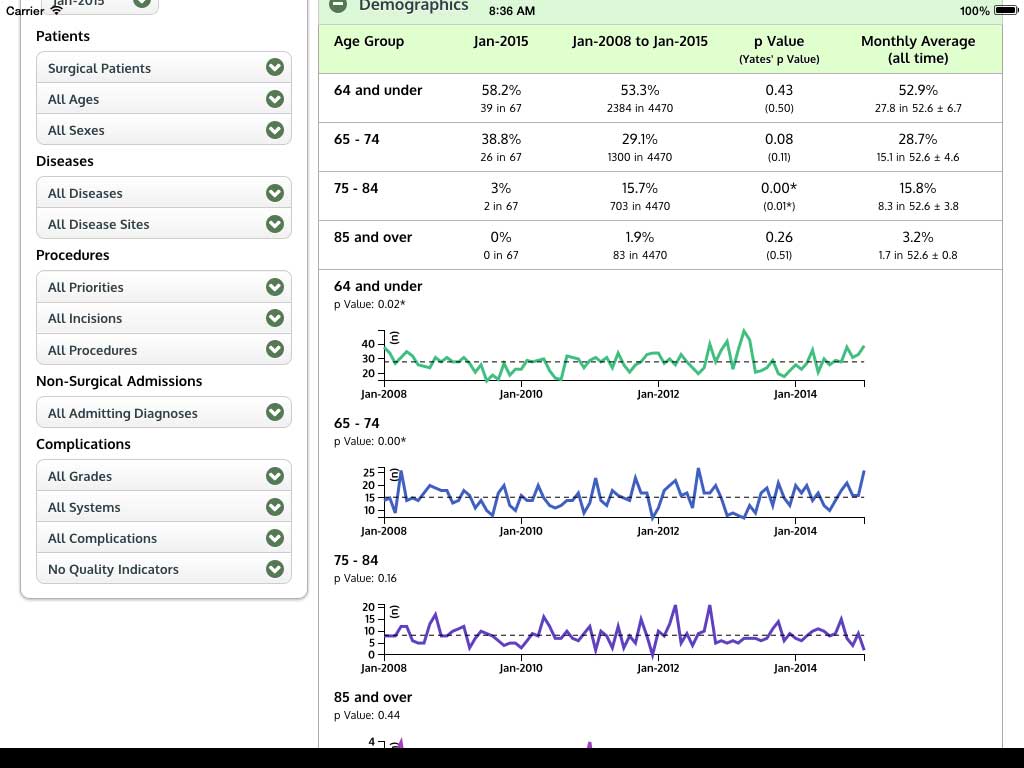
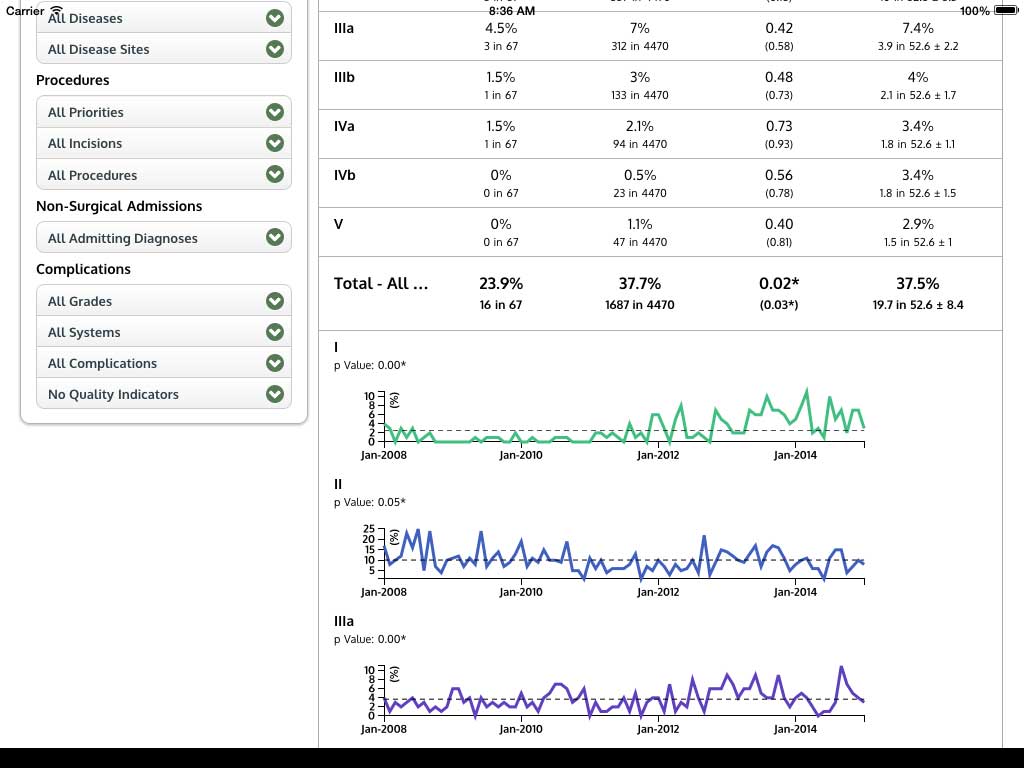
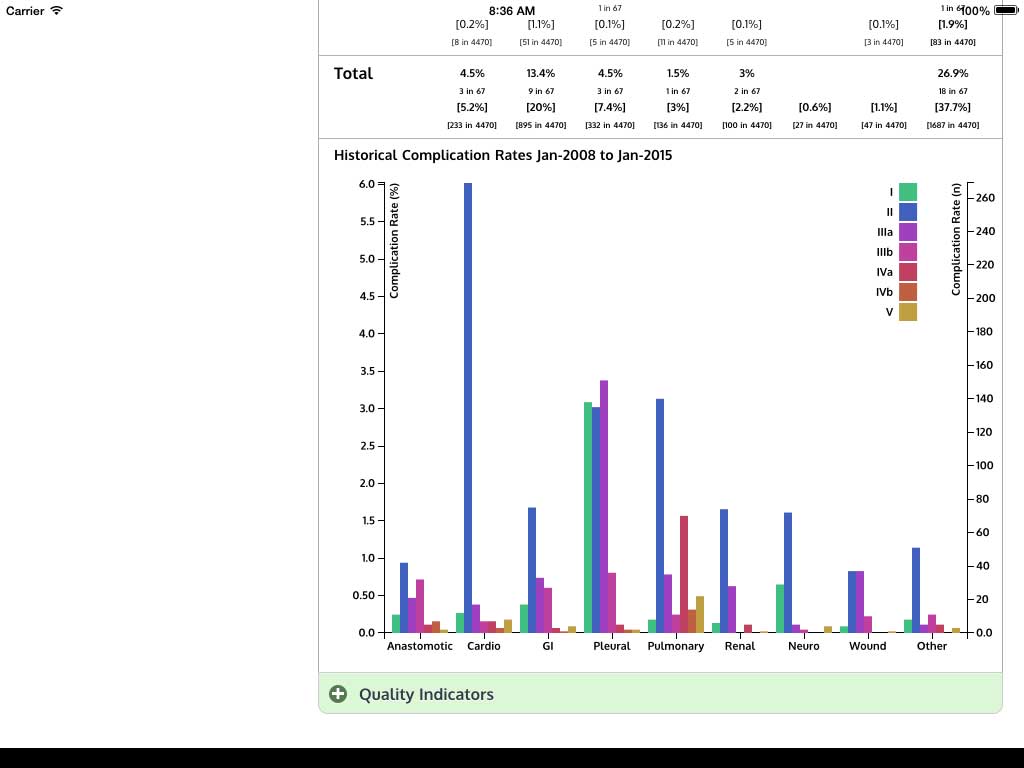
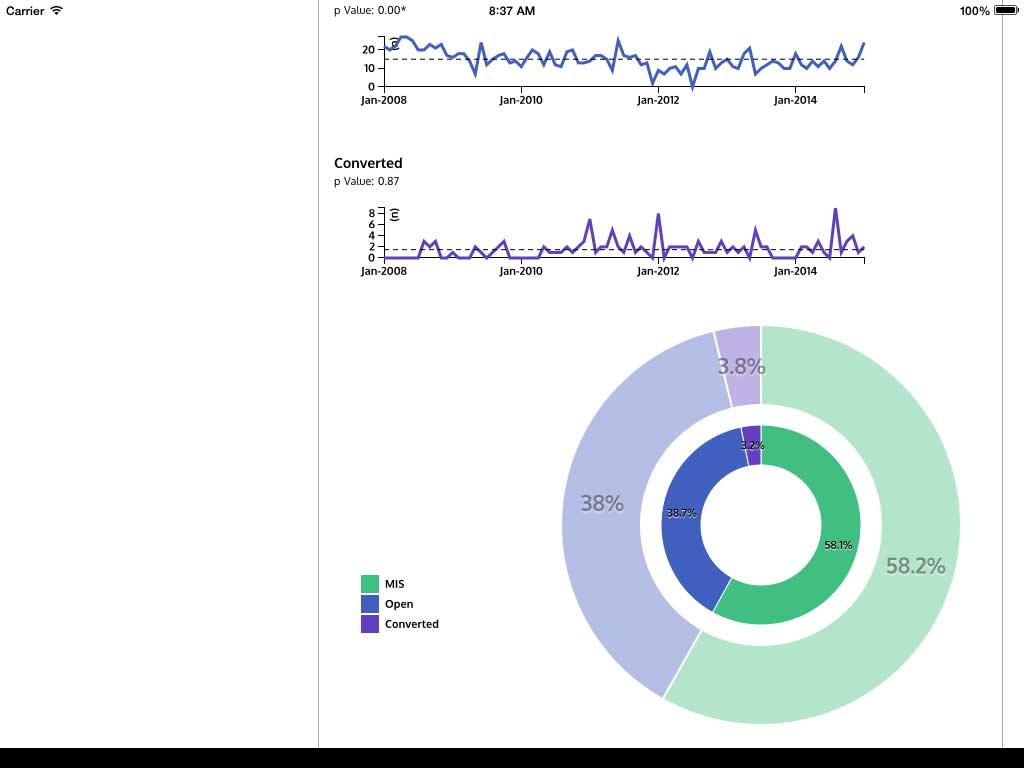
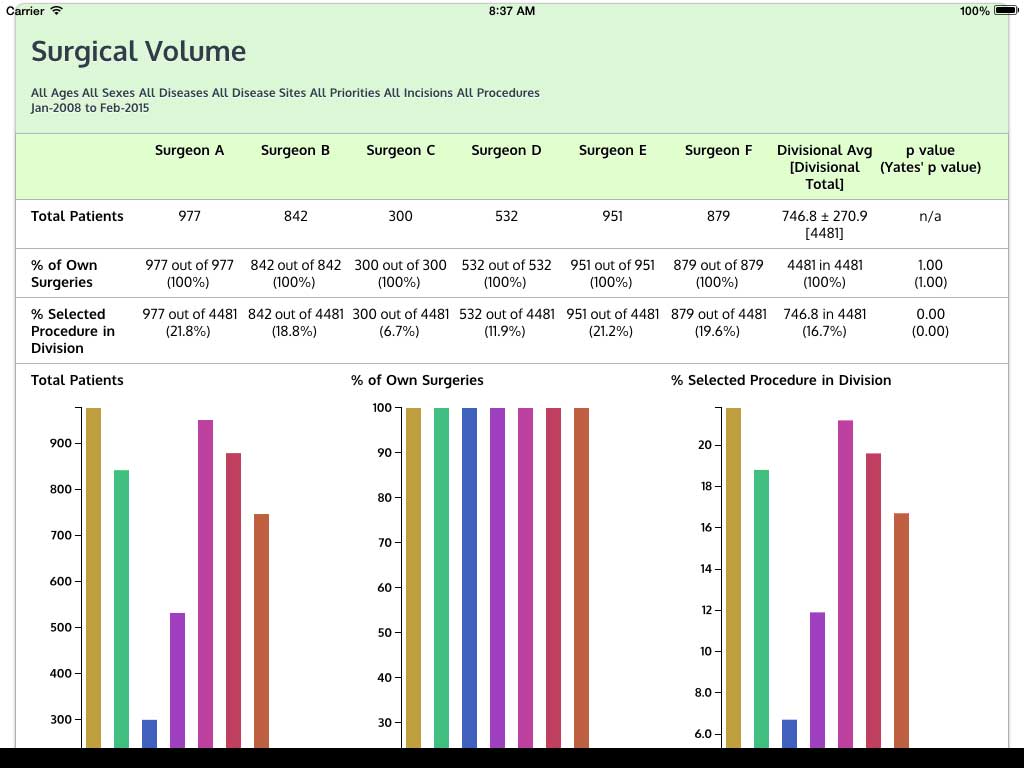
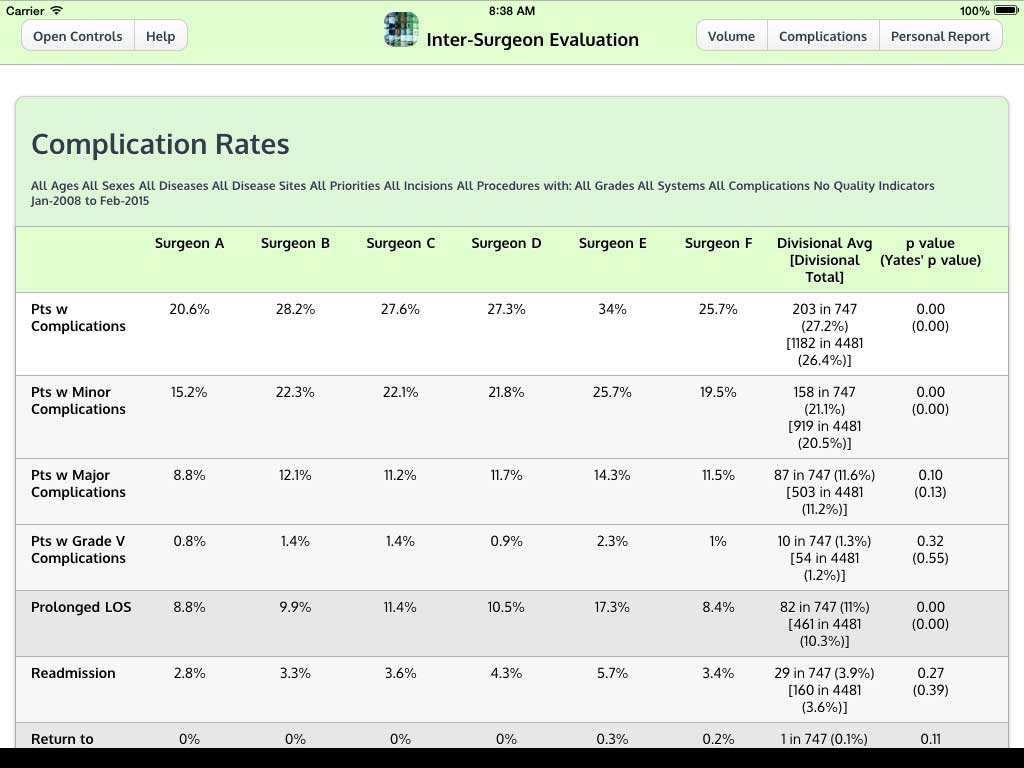
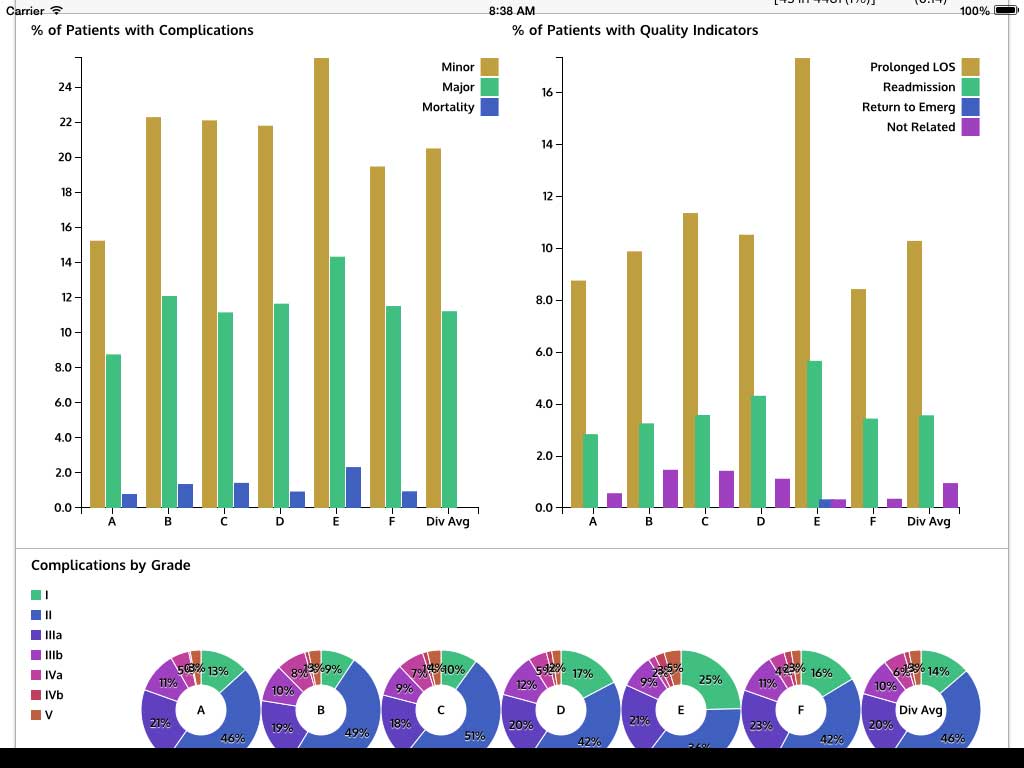
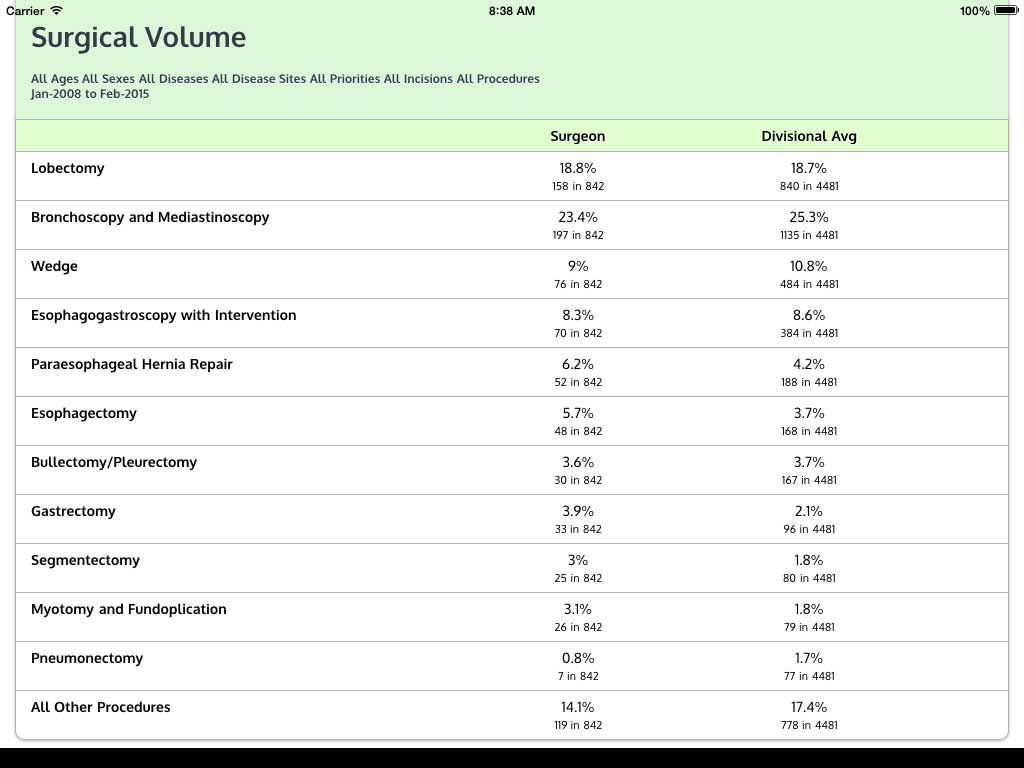
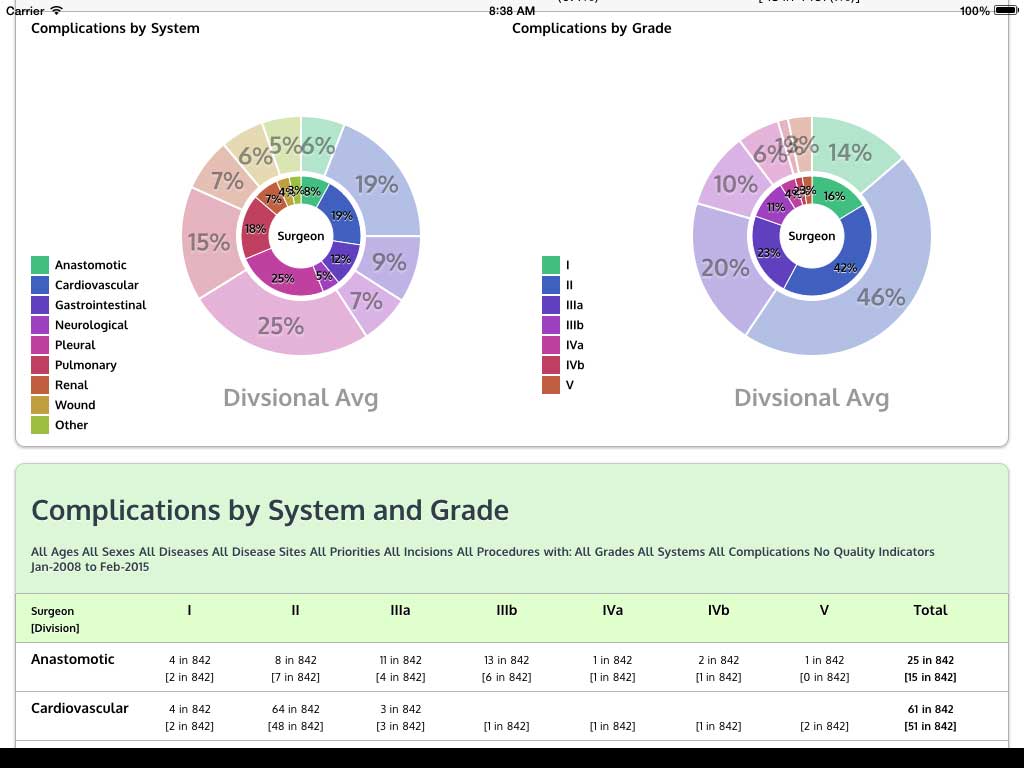
Ivanovic J, Anstee C, Finley C, Gilbert S, Maziak DE, Shamji FM, Sundaresan RS, Villeneuve PJ, Ramsay T, Seely AJE. Development and implementation of the Thoracic Surgery Quality Monitoring, Information Management, Clinical Documentation System for continuous, point-of-care reporting and evaluation of post-operative adverse events.
Ottawa Hospital Research Institute (OHRI) Annual Research Conference, November 2014. Ottawa, Ontario.
Ivanovic J, Anstee C, Ramsay T, Gilbert S, Maziak DE, Shamji FM, Sundaresan RS, Villeneuve PJ, Seely AJE.
Development, implementation, and qualitative evaluation of surgeon-specific outcome reports and of a surgeon-led, continuous quality improvement program in thoracic surgery.
10th Annual Ottawa Hospital Patient Safety Conference, October 2014. Ottawa, Ontario.
Ivanovic J, Seely AJE, Kwok ESH, Barlow-Krelina E, Frank JR, Cwinn A, Worthington J, Calder LA. Enhancing Thoracic Surgery M&M Rounds – Incorporating a Structured Model into a Standardized Adverse Event Classification System.
10th Annual Ottawa Hospital Patient Safety Conference, October 2014. Ottawa, Ontario.
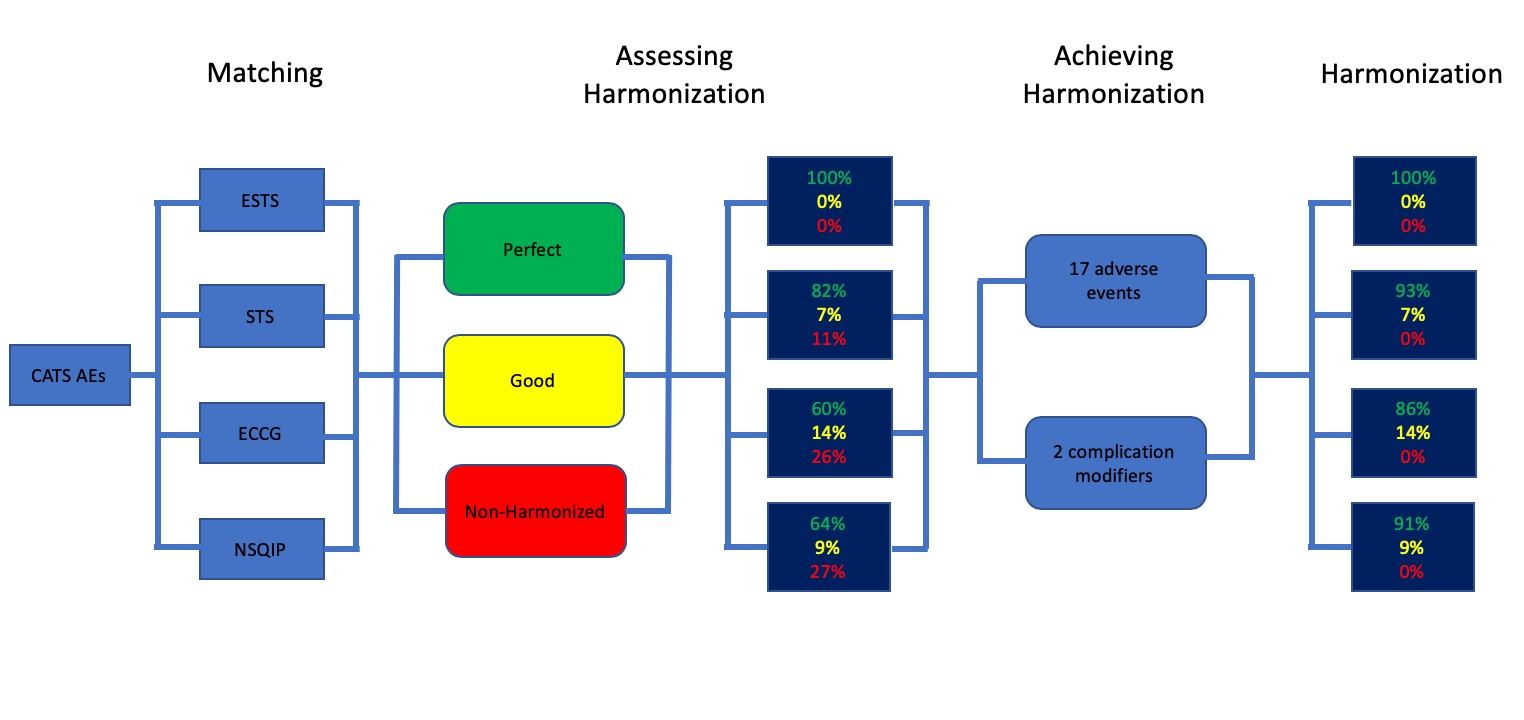
Ivanovic J, Seely AJE, Anstee C, Villeneuve PJ, Gilbert S, Maziak DE, Shamji FM, Forster AJ, Sundaresan RS. Measuring surgical quality: A comparison of postoperative adverse events with the American Colle ge of Surgeons National Surgical Quality Improvement Program and the Thoracic Morbidity and Mortality System.
9th Annual Ottawa Hospital Patient Safety Conference, October 2013. Ottawa, Ontario.
Ivanovic J, Ramsay T, Maziak DE, Gilbert S, Shamji FM, Sundaresan RS, and Seely AJE. Improving patient care through the development and evaluation of the Thoracic Surgery Quality Monitoring, Information Management, and Clinical Documentation (TSQIC) System.
8th Annual Ottawa Hospital Patient Safety Conference, November 2012. Ottawa, Ontario.
Ivanovic J, Khan Z, Anstee C, Ramsay T, Maziak DE, Gilbert S, Shamji FM, Sundaresan RS, and Seely AJE. Improving patient care through implementation of the Thoracic Surgery Quality Monitoring, Information Management, and Clinical Documentation (TSQIC) System.
Ottawa Hospital Research Institute (OHRI) Annual Research Conference, November 2011. Ottawa, Ontario.